Lymphoblasts: Their Definition, Properties, and Role in the Human Body
The immune system, a complex network of cells, proteins, tissues, and organs, constantly defends the human body against daily threats from germs and microorganisms. While primary defenses like skin and mucous membranes block entry, an intricate internal system protects us once pathogens are inside. Crucially, lymphoblasts are the precursors to lymphocytes, the essential immune cells.
What is a Lymphoblast?
A **lymphoblast** is an **immature cell** that serves as a precursor to **lymphocytes**, which are cellular components responsible for the body’s tertiary immunological barriers. However, a significant terminological conflict exists, as the term “lymphoblast” also refers to a lymphocyte that has enlarged after being stimulated by an antigen. Both events are distinct, yet share the same name.
1. The Lymphoblast as a Precursor
In healthy individuals, lymphoblasts (understood as cells giving rise to lymphocytes) are typically found in the **bone marrow** of long bones. If “lymphoblast” is taken as a progenitor, it can be interchanged with “common lymphoid progenitor,” as both ultimately lead to prolymphocytes, the intermediate form that matures into the desired cell type.
Generally, the transformation from a lymphoblast to a functional lymphocyte follows these steps:
- The maturation of lymphoblasts or common lymphoid progenitors in the bone marrow leads to commitment to either the B or T lymphocyte lineages.
- Immature lymphocytes proliferate through various stages during their maturation to ensure an adequate supply of cells that will later mature.
- Lymphocytes are selected through multiple steps during their maturation to conserve specificities useful for each occasion, based on the expression of intact antigen receptor components and what they recognize.
At the culmination of this process, **lymphocytes** possess receptors for specific antigens, enabling them to produce **antibodies** and destroy abnormal cells, such as germs and other pathogens. These cells account for approximately 30% of total leukocytes in peripheral blood and represent the tertiary **immune barriers**. **T lymphocytes** directly act upon and destroy pathogens, while **B lymphocytes** detect antigens (foreign substances, presumably from pathogens) and generate specific antibodies, which cause the invading microorganism to lose its pathogenicity.
2. The Lymphoblast as a Lymphocyte with Altered Morphology
On the other hand, and despite the potential for confusion, a **lymphoblast** also refers to a **lymphocyte that has enlarged after being stimulated by an antigen**. In this scenario, upon recognizing the antigen, this type of white blood cell becomes activated, leading to growth in its cytoplasm and nucleus, and an increase in messenger RNA and specific protein production. This large lymphoblast then begins to divide 2-4 times every 24 hours for 3-4 days, producing up to 1000 clones of the original lymphocyte. Each clone exhibits specificity for the same antigen that initially activated it. Finally, the resulting cells can differentiate into specific cell types that will combat the pathogen in various ways.
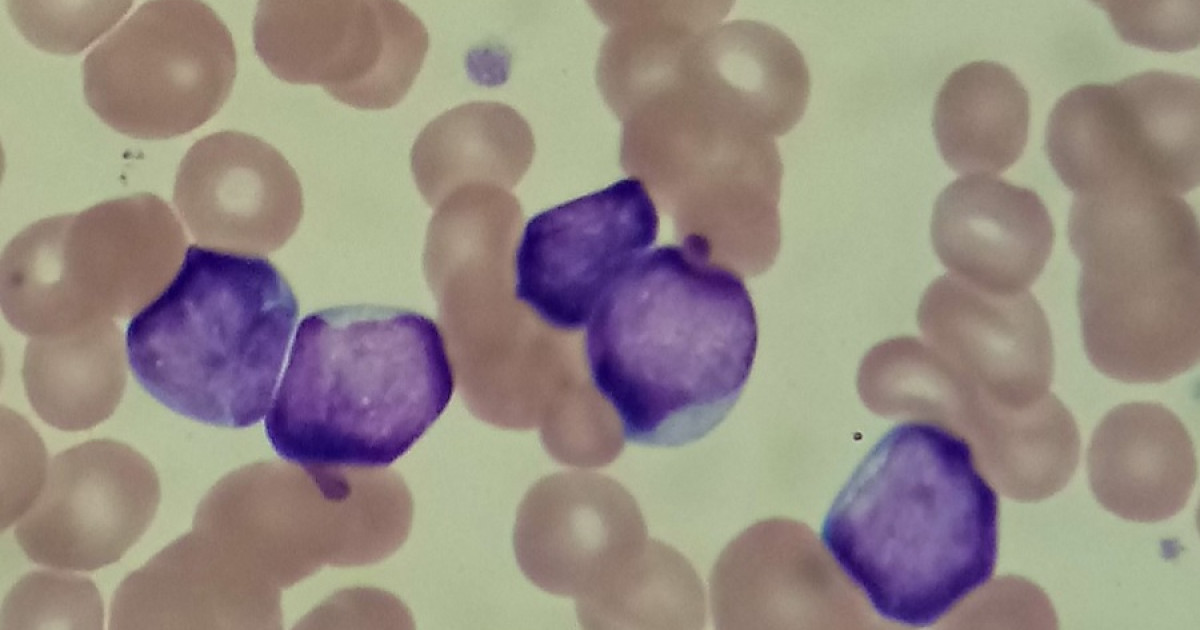
Characteristics of a Lymphoblast
While we’ve explored what a lymphoblast is, understanding its **morphology** provides a general overview of this unique cell:
- Size: Lymphoblasts range in size from 10 to 18 micrometers.
- Shape: They are typically rounded or oval.
- Internal Structure: They feature a single cell nucleus and a bluish cytoplasm, which may contain granulations.
- Nucleus-to-Cytoplasm Ratio: The ratio typically falls between 5:1 and 7:1.
- Nucleoli: They usually present 1 to 2 nucleoli, regions within the cell nucleus responsible for the production and assembly of ribosomes.
Acute Lymphoblastic Leukemia (ALL)
**Leukemias** are cancers originating in the cells that would normally differentiate into various types of blood cells, specifically B and T lymphocytes. When there is an uncontrolled proliferation of lymphoblasts (understood as lymphocyte precursors), these invade the **bone marrow**, preventing the production of other crucial cells like red blood cells and platelets. This severe condition is known as **Acute Lymphoblastic Leukemia (ALL)**.
ALL affects individuals of any gender, ethnicity, or age, though it is relatively uncommon. The incidence in the United States is approximately 1.7 cases per 100,000 people annually. Despite its rarity, ALL is the most common type of cancer in children under 20, accounting for a significant percentage of leukemias in this age group.
ALL is triggered by the mutation of a single lymphoblast in the bone marrow, but researchers have not yet pinpointed the exact cause of this event. However, several risk factors can increase the likelihood of developing ALL:
- Genetic factors: Individual chromosomal alterations determined at birth.
- Radiation exposure: Exposure to X-rays or ionizing radiation before or after birth.
- Previous chemotherapy: A history of certain chemotherapy treatments.
- Viral infections: Infection with specific types of retroviruses.
- Chemical exposure: Contact with certain chemicals, such as benzene and some alkylating compounds.
The prognosis for ALL can be encouraging, particularly for younger patients. The 5-year survival rate for patients under 20 diagnosed with ALL is very high, often exceeding 90%. However, for patients over 20, the 5-year survival rate is considerably lower, typically below 40%. These figures are estimates, as each case depends on the individual’s physiological characteristics and disease progression.
A Terminological Confusion
As indicated by various sources, the definition of a **lymphoblast** can be a point of confusion. Many define it as a **lymphocyte that has become larger after being stimulated by an antigen**. These activated cells may appear as immature lymphocytes and were once thought to be precursor cells. Conversely, numerous other authoritative sources utilize “lymphoblast” to mean the precursor cell that transforms into a prolymphocyte, which then gives rise to the well-known B and T lymphocytes responsible for immune responses. This terminological overlap highlights that, in some specific instances, the medical terminology for a particular event or cell type may not be consistent across all consulted sources, necessitating contextual understanding.